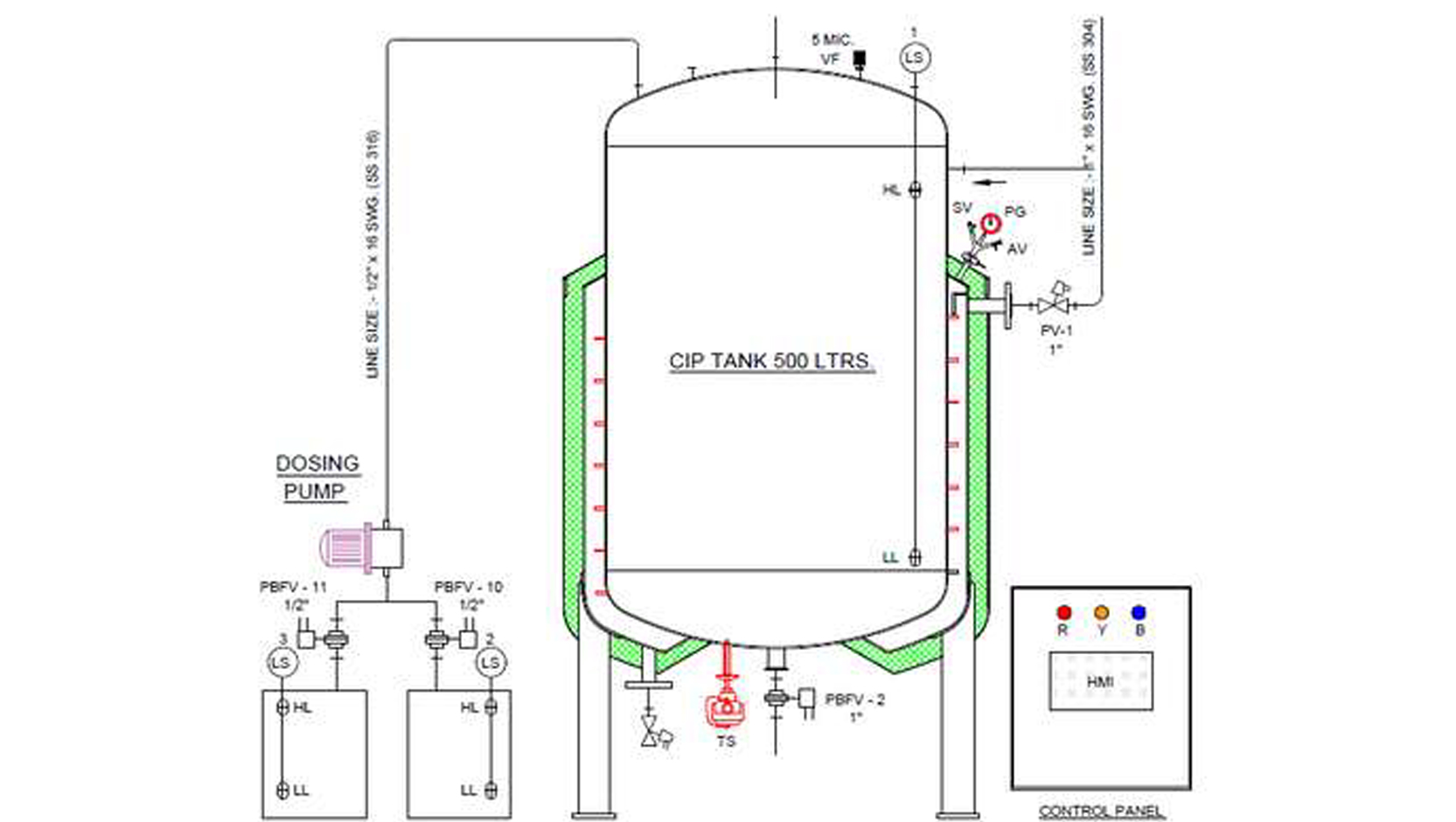
Cleaning-In-Place (CIP) and Sterilization-In-Place (SIP) are systems designed for automatic cleaning and purification without major disassembly and assembly work. We design, develop, manufacture, supply and install Mobile CIP/SIP Skid and Fixed these Units for sanitization and sterilization. The units are custom-made, modular, skidded in automated or semi-automated Models as per the required time cycle for cleaning and sterilization as a part of cGMP requirements, from adjustable to large fixed Multi-Tank systems.
Kyra’s CIP/SIP skid is ideal for industries where hygiene, sterility, and repeatable cleaning are critical:
1. Expertise in Pharma Process Equipment
As a Manufacturer & Exporter & Supplier in Mumbai, India, Kyra International has great engineering experience in building sip process skids for regulated industries.
2. Customization for Your Needs
We work with you to design the skid based on your process flow, number of cleaning circuits, cycle time, and validation requirements.
3. Pre-Commissioning & Testing
Our CIP/SIP skids are fully built, wired, and FAT (Factory Acceptance Tested) before delivery, ensuring quick and smooth installation at your site.
4. Validation Support
We support DQ, IQ, OQ, and PQ documentation and help set up validation protocols (conductivity checks, riboflavin tests, etc.).
5. Service & Maintenance
Our Mumbai-based support team provides installation, commissioning, training, and periodic maintenance, minimizing your risk and maximizing uptime.
A “CIP/SIP skid” is a modular frame-mounted system that automates Clean-in-Place (CIP) and Sterilize-in-Place (SIP) operations for process equipment, like tanks and piping, without needing to disassemble them. .
Automated CIP/SIP significantly reduces downtime, minimizes human error and pollutants risk, provides repeatable and validated cleaning cycles, and helps meet regulatory standards.
Yes, Kyra’s skid supports validation protocols, including riboflavin tests for CIP, temperature mapping for SIP, and generates batch records for regulatory standards.
We use SS 316L stainless steel for all product-contact surfaces, polished or electropolished, and orbital welds for hygienic joints.
Yes. We offer fully automated skid-installed systems with PLC + HMI control, cycle recipe management, and data recording.
Definitely. The skid is designed for effective use of water, detergent, and steam; closed-loop recirculation reduces waste, and optimized cycles reduce utility consumption.
The closed-loop skid design cleans only the intended circuits, controls concentration and flow, and sterilizes lines with steam to reduce remaining microbes.
Yes. We can design the skid for single or multiple cleaning circuits, depending on how many vessels or lines you need to clean, and whether you want centralized or decentralized cleaning.
Kyra International’s CIP/SIP Skid isn’t just a piece of equipment; it’s your partner in maintaining hygiene, efficiency, and regulatory compliance throughout your manufacturing process. Whether you run a sterile injectable facility, a biotech plant, or a multi-product cosmetic line, our skid delivers validated cleaning cycles, repeatability, and safety, all supported by our engineering team in Mumbai, India.
Let’s talk about your cleaning needs. Reach out to us for a detailed design, FAT, and validation plan, and let’s bring clean, sterile production to your facility.